CDC: Some immunocompromised people may need 4th COVID-19 vaccine dose
WASHINGTON - People with certain health conditions that make them moderately or severely immunocompromised may receive a fourth COVID-19 vaccine dose, according to updated guidance from the U.S. Centers for Disease Control and Prevention.
Those who completed their primary series of an mRNA COVID-19 vaccine by either Pfizer-BioNTech or Moderna, and received an additional third mRNA dose, may receive a fourth dose at least six months later, the agency said.
In August, the U.S. authorized a third vaccination dose for American adults at high risk from COVID-19 because of severely weakened immune systems. The Food and Drug Administration ruled that transplant recipients and other similarly immune-compromised patients can get a third dose of either the Pfizer or Moderna vaccine at least 28 days after their second shot — making a third dose part of their initial prescription.
The Pfizer and Moderna vaccines offer powerful protection for otherwise healthy people, but many who take immune-suppressing medications or have diseases that tamp down their immune systems generally get less benefit from the standard two doses. The CDC previously cited one study suggesting about 40% to 44% of people hospitalized for a so-called breakthrough case — infection after vaccination — are among the immune-compromised.
In its updated guidelines, the CDC says in some situations, "people who are moderately and severely immunocompromised may receive a total of four COVID-19 vaccine doses."
"A person who is moderately or severely immunocompromised and has received two doses of an mRNA vaccine and ≥28 days has elapsed since the second dose, should receive an additional mRNA dose immediately, followed ≥6 months later by a single COVID-19 vaccine booster dose," the CDC says.
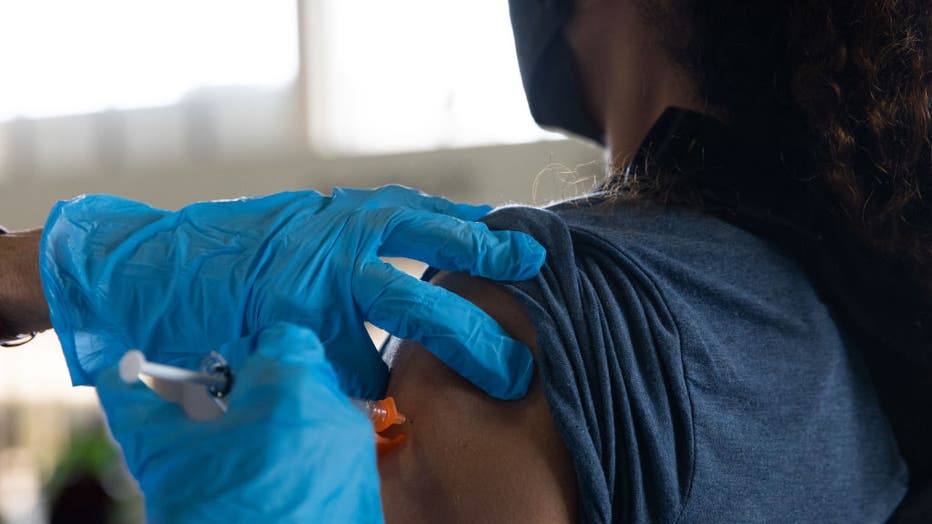
FILE - A patient receives a booster dose of the Pfizer-BioNTech COVID-19 vaccine during an Oakland County Health Department vaccination clinic at the Southfield Pavilion on Aug. 24, 2021, in Southfield, Michigan. (Photo by Emily Elconin/Getty Images)
U.S. regulators have also already signed off on extending COVID-19 boosters to all Americans 65 and older and other high-risk groups, including those who live and work in settings that put them at an increased risk to be exposed to the virus. This also includes those who received the Moderna or Johnson & Johnson vaccine.
Furthermore, anyone eligible for an extra dose can get a brand different from the one they received initially.
RELATED: COVID-19 vaccines: CDC OKs Moderna, J&J and mix-and-match boosters
The CDC says that moderately and severely immunocompromised adults who received the single-dose J&J shot should receive a single COVID-19 booster vaccine (Pfizer-BioNTech, Moderna or J&J) at least two months later.
But the agency notes: "A person who received one primary dose of Janssen COVID-19 vaccine should not receive more than two COVID-19 vaccine doses."
While the CDC does state that a fourth dose may be given to some immunocompromised people, the CDC does not have an official recommendation for receiving both a booster shot and an additional dose — and patients should consult with their doctor.
This story was reported from Cincinnati. The Associated Press contributed.


